LabLynx KB:SysAdmin - 4.4 security system setup
|
|
This is an article specific to the Category:LabLynx knowledge base. Its context outside of LabLynx, Inc may not be apparent, thus why it appears inside the LabLynx KB namespace. |
Security system setup
Managing security in installed LIMS software is a vital step, ensuring the integrity of the data collected, analyzed, and stored in your laboratory is maintained. This document continues to discuss security, addressing the concepts of document types and records.
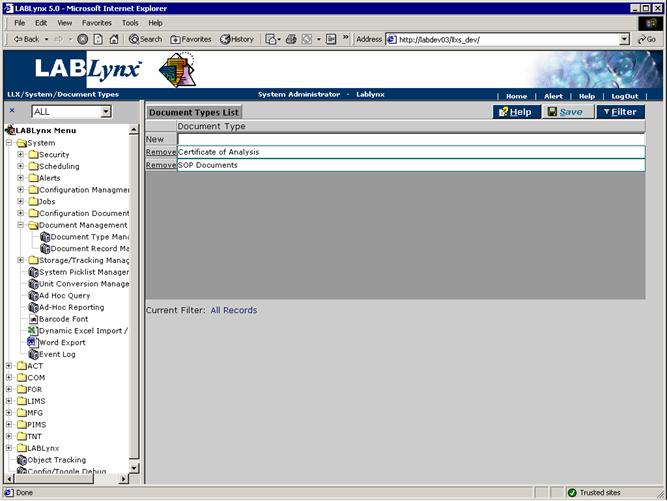
Document type management
Document types are grouping entities under which actual documents are being tracked in the revisioning module. Document types are used in the system to group documents being tracked for revision. In addition, the system uses the type of Document to display certain documents in specific areas of the system (i.e. documents are filtered on by type):
The user will see a list of currently defined document types being tracked in the system. The User may select an existing type by clicking on the document type name, which will allow the editing of the selected record. The user may choose to create a new document type by simply entering the name of the new type into the document type entry area next to the New button in the list. Once entered the user simply has to click on the Save button.
(Note: Do not delete document types that are installed with the system as certain modules within the system have links to this area.)
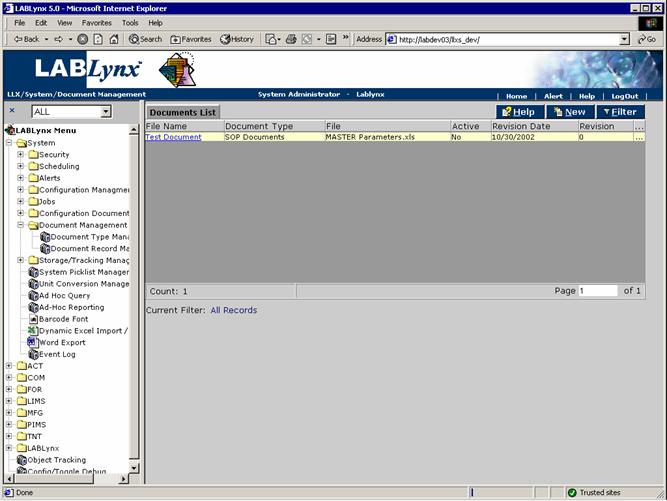
Document record management
Documents are files that may be uploaded to the server for preservation and tracked by the system. Documents are used throughout the system in various modules and are stored on the server in order to prevent un-authorized modifications to them. If users try to modify an existing document, they can download it locally; however, if they try to upload it, the system will force a revision.
As an example, a standard operating procedure (SOP) for a test is based on specific methodology (American Society for Testing and Materials [ASTM] perhaps). When ASTM revises their methodology, it stands to reason that the laboratory may have to modify their SOP as well. The original SOP can be downloaded from the system, modified, and then re-uploaded, which will cause the revision information for that SOP to increment. This way, the laboratory will have an online history for the SOP.
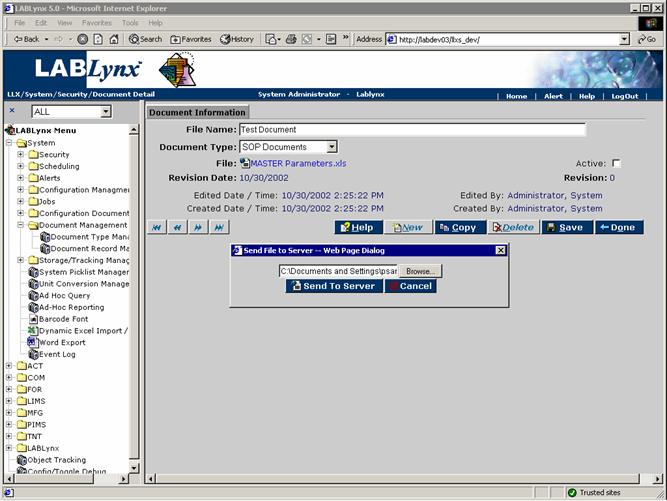
The user will see a list of currently defined documents in the system regardless of type. The user may edit an existing document by clicking on the URL in the File Name field. Alternatively, the user may select the New button in the Documents List, which will cause the system to re-direct to the Document Information screen:
The Document Information screen displays the name, filename, document type, status, and revision information to the user. The user may edit the name, type, and status of a document, but any attempt to upload a new file on an active document will cause the revision number to increment: