LabLynx KB:SysAdmin - 5.0 LIMS system setup
|
|
This is an article specific to the Category:LabLynx knowledge base. Its context outside of LabLynx, Inc may not be apparent, thus why it appears inside the LabLynx KB namespace. |
LIMS system setup
The testing that a laboratory performs is highly dependent on the workflow, standard operating procedures, and business practices that are adhered to. Because of this, LabLynx designed the LIMS test set-up area to be highly configurable in order to meet the requirements of small, medium, and large laboratory facilities. This document aims to discuss setup of the LIMS system, addressing the concepts of master parameters and processes.
Master parameter
Master parameters are added to the LIMS in order to have consistent values for parameter names in the test setup section. Master parameters are parameters for which system users will enter results. They are routinely assigned to tests, though they serve other functions within the system as well. All parameters for which the LIMS will store information are required to be defined as master parameters. This is true whether the actual parameters are reportable.
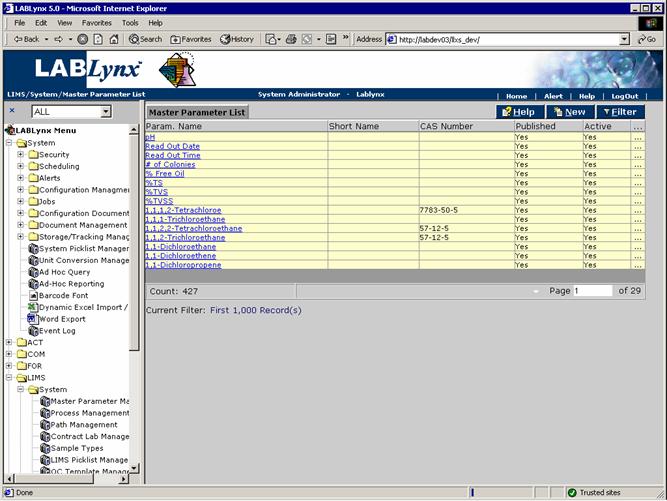
The Master Parameter List displays the parameter name, parameter short name, the CAS number, active, and published columns:
For example, Arsenic is a parameter is routinely analyzed and should be listed in this section. Alternatively, values such as oven temperature, spike % Recovery, spike/duplicate RPD, observation, finding, date, reagent, result, and any other data that requires capture should be listed. Do not view master parameters as just a list of target parameters from instruments; the system can accommodate much more diverse parameters, and it is in the best interest of the laboratory to explore their uses.
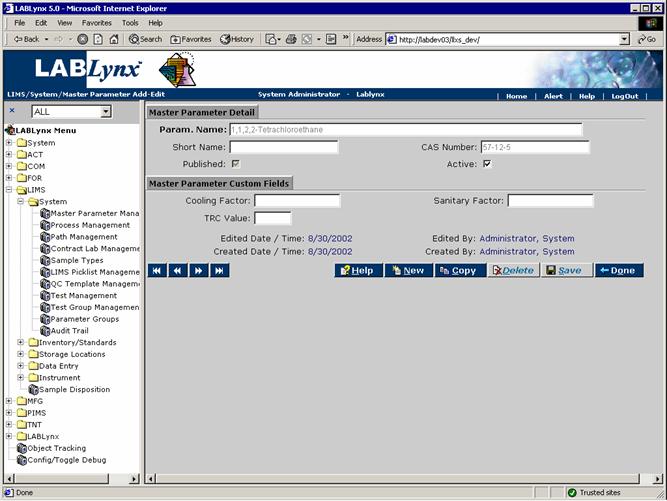
Clicking on the parameter name or the New button takes the user to the Master Parameter Detail screen:
From this screen, the user defines many aspects of the parameter. Fields in bold are required. The various fields are explained below:
| Field | Description |
|---|---|
| Param. Name | This is the user-defined value for the formal name of the parameter. |
| Short Name | This is the user-defined abbreviation for the parameter. |
| CAS Number | This is the user-defined Chemical Abstract Services registry number. |
| Published | This is a flag that determines whether or not to publish the parameter. |
| Active | This is a flag used by the system in order to determine whether or not to make this parameter available for use. |
| Edited Date / Time | This is the date and time of the last edit. |
| Edited By | This is the system user who last edited the entity. |
| Created Date / Time | This is the date and time of creation for this item. |
| Created By | This is the system user who created this item. |
Process
Each process is merely a named point in time at which one or more samples may be grouped together by a specific subset of system users into a batch. Processes that exist within the laboratory are ultimately for assignment to tests that model the appropriate workflow within the laboratory. In addition to workflow definitions, each process may be assigned to all system users within the system, or the process may be assigned to a subset of system users.
Processes should be thought of as points at which sample tests may be grouped together because they share common requirements. Examples of processes may be organic extractions, metals digestions, wet chemistry, ion chromatography, and countless others. These examples are simply for illustration as processes may be much more detailed and numerous or, conversely, more generic and fewer in number. The concept is that the individual responsible for the creation/definition of the laboratory processes should be familiar with all aspects of the laboratory and be able to analyze the flow of current lab testing as well as translate those requirements into their respective processes.
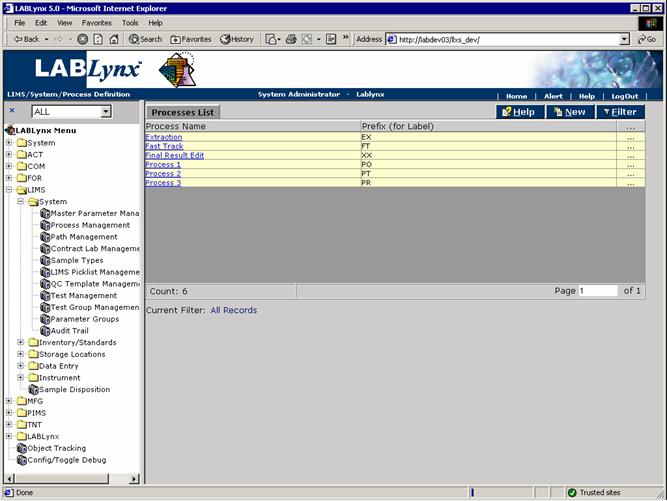
The Processes List shows currently defined processes. The list includes the Process Name and Prefix:
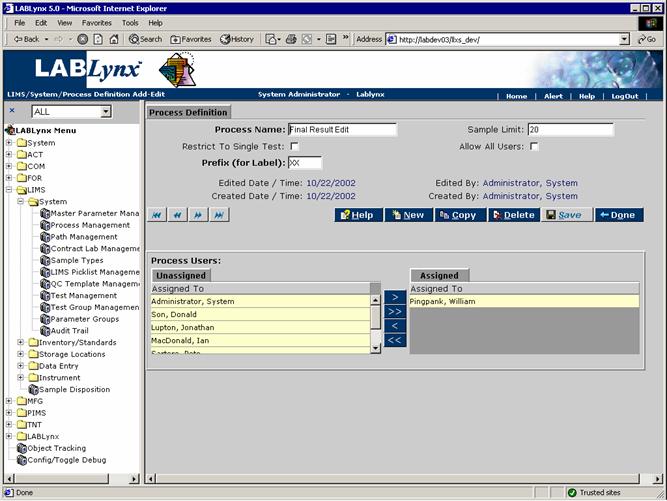
The user may edit an existing record simply by clicking the URL of the desired record in the Processes List. The user may also create a new record in this list by selecting the New button, which will redirect to the Process Definition screen:
From this screen, the user defines many aspects of the process. Fields in bold are required. The various fields are explained below:
| Field | Description |
|---|---|
| Process Name | This is the user-defined value for the formal name of the process. |
| Sample Limit | This is the user-defined value for the maximum number of samples that may be grouped together into one batch for the process. |
| Restrict To Single Test | This is a flag to prevent dissimilar tests from being added to a process group or batch. |
| Allow All Users | This is a user-defined flag indicating that all system users will have the security rights to view and create batches for the process. |
| Prefix (for Label) | This is a set of two user-defined characters appended to the beginning of the batch number for this process. |
| Edited Date / Time | This is the date and time of the last edit. |
| Edited By | This is the system user who last edited the entity. |
| Created Date / Time | This is the date and time of creation for this item. |
| Created By | This is the system user who created this item. |